Diclofenac Sodium Injection
Indication
Usage and dosageÂ
Deep intramuscular injection. One 75mg (one), 2 or 3 times a day.
ContraindicationsÂ
1. Patients with an allergic or asthmatic history of aspirin or other non-steroidal anti-inflammatory drugs are prohibited.
2. Pregnant and lactating women are disabled.
Pharmacology and toxicology
1. Pharmacology
Diclofenac sodium is a non-steroidal anti-inflammatory analgesic derived from phenylacetic acid. Its mechanism of action is inhibition of cyclooxygenase activity, thereby blocking the conversion of arachidonic acid to prostaglandin. At the same time, it can also promote the combination of arachidonic acid and triglycerides, reduce the concentration of free arachidonic acid in cells, and indirectly inhibit the synthesis of leukotrienes.
Diclofenac sodium is a potent non-steroidal anti-inflammatory drug. It has a stronger inhibitory effect on prostaglandin synthesis than aspirin and indomethacin.
2. Toxicology
Diclofenac sodium was given to rats daily at 2 mg/kg. No long-term observations revealed an increase in the incidence of tumors. In a two-year study of mice, 2 mg/kg of drug was administered daily, and no tumor proneness was observed. Various mutation studies have not found diclofenac-induced gene mutations. The rats were treated with 4 mg/kg daily, and no infertility occurred in males and females.
Acute toxicity test results: The oral LD50 of rats is 150 mg/kg; the oral LD50 of mice is 390 mg/kg.
Shelf LifeÂ
36 monthsÂ
StorageÂ
Seal storage. The formulated liquid is for single use only and must not be frozen or refrigerated. Â
Product Photo


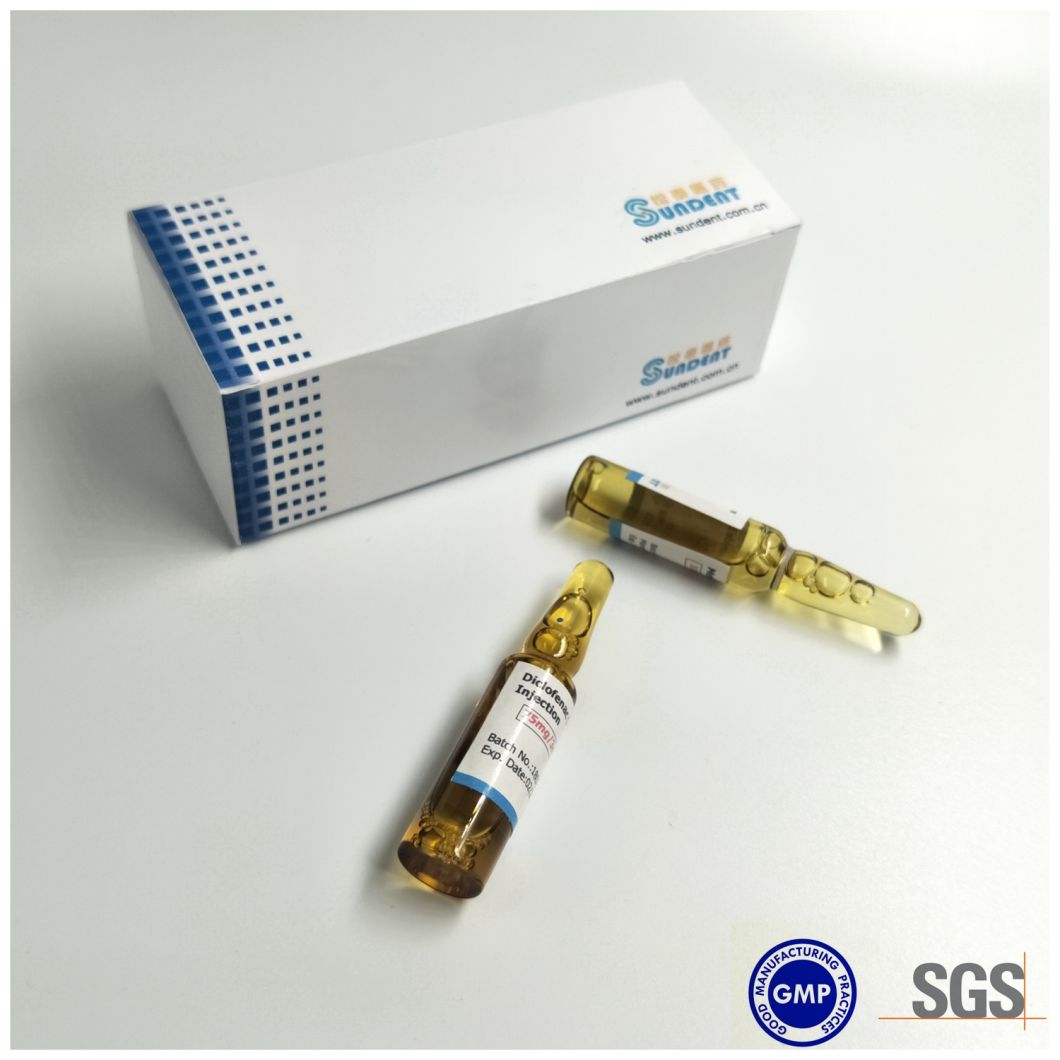
Â
Our factory
This product is produced in our GMP factory, all our facility has past China GMP, and also officially certificated by more than ten countries' MOH.
Our Service
We can specially develop the products per customers' specification.
We can offer OEM service with customers' private brands and labels.
We can offer excellent after-sale service and professional technical support.
Our Quality control system
In manufacturing, quality is what sustains our life.  Starting from the material selection, there are many factors that contribute to the quality control process.  Our manufacturing facility is GMP certified. Quality Control (QC) and monitoring procedures are built into every aspect of our work.
Our Quality Control System is outlined below:
- Initial inspection (such as raw material, excipients, package materials, etc.)
- In-process inspection (make sure that each testing items should be qualified )
- Final inspection before shipment of each order.Â
- Provide temperature control recorder when it is necessary for special market before loading.
Our Registration Team and technical support
We have experienced team to provide qualified and professional registration documents for most of countries. Up to present, we have registered more than hundred products and exported to more than 20 countries. Especially including those in Asia, Africa, South America, Middle east area and East Europe.
We can provide regulatory documents
- GMP Certificate /Â CE Certificate
- Free sales certificateÂ
- Certificate of Pharmaceutical Products
- Manufacture License
- CTD Dossier.Â
Supply Meat Bone Meal,Supply Wheat Gluten Meal,China Corn Gluten Meal
Wudi Deda Agriculture Co., Limited , http://www.challmeal.com