Model NO.: YD180
Logo Printing: With Logo Printing
Medical Device Regulatory Type: Type 3
Medical Devices Reg./Record No.: Ssyjxzz-2001-0249
Style: OEM
Lenght: 1.3 or 1.5 M
Trademark: IU OR OEM
Transport Package: 1PC Per Bag. 500 Per CTN. 800CTN /40hq
Specification: according to customer′s request
Origin: China
HS Code: 90183900
Model NO.: YD180
Logo Printing: With Logo Printing
Medical Device Regulatory Type: Type 3
Medical Devices Reg./Record No.: Ssyjxzz-2001-0249
Style: OEM
Lenght: 1.3 or 1.5 M
Trademark: IU OR OEM
Transport Package: 1PC Per Bag. 500 Per CTN. 800CTN /40hq
Specification: according to customer′s request
Origin: China
HS Code: 90183900
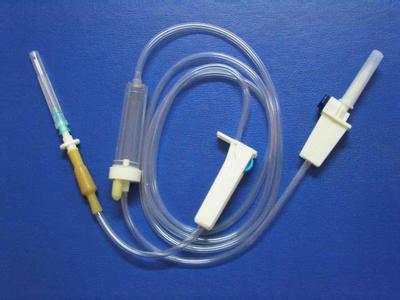
Disposable Steriled  IV Infusion Set (Model  YD180)INFUSION SET with two plastic inserters two adjustors
Item No: YD180
This is disposable infusion set with two plastic inserters and two adjustors.
Assesoris: Vented spike, dripping champer, roller clamp, solution filter, emulsion tube.
Features:
1) For single use
2) Transparent drip chamber with filter
3) Flexible tubing with standard length of 135cm
4) Thick latex connector
5) Luer lock adapter with hypodermic needle
6) Sterilization: Ethylene-gas-sterilization
| Name of Sample | Disposable Infusion Set with needle | Type | IS-VZ  0.7 | ||||
| LOT NO | 20130618 | Testing Standards | contract requirements | ||||
| Sterilization Lot | 20130618 | Quantity of Tested Batch | 1260000 pcs | ||||
| Quantity Of Sample | 50pcs | Testing Date | SEP 7, 2013 | ||||
| Type | Testing items | Standard Requirement and Standard Term and Conditions | Actual Testing Result | Conclusion | |||
| Biological Properties | Sterility | The infusion set in individual package should be sterile (8.2) | Missing | / | |||
| Pyrogen | Infusion set has no pyrogen (8.3) | Missing | / | ||||
|  Chemical Properties |
Reduction Matter | Difference of volumes of potassium permanganate [ c(kmno4 = 0.002mol/L)] consumed by test liquid and blank liquid should be not more than 2.0 ml (7.1) | 0.5ml | Eligible | |||
| Ph | The PH difference between testing liquid and blank liquid is not more than 1.5 (7.3) | 0.19 | Eligible | ||||
| Ultraviolet Absorbance | The absorbance of testing liquid should be not more than 0.1 (7.5) | 0.046 | Eligible | ||||
| Ethylene Oxide Residuum | The ethylene oxide residuum should be not more than0.5mg; Unit: mg  (7.6) |    0.29 | / | ||||
|  Physical Properties |
Particle Content | For the number of 15-25min particles in 200ml eluent, not more than 1 particle/ml | Eligible | Eligible | |||
| For the number over 25min particles, not more than 0.5 particle/ml (6.1) | Eligible | ||||||
| Sealing Property | No air leakage of infusion set (6.2) | Eligible | Eligible | ||||
| Connection Intensity | Connection of parts for liquid channel of infusion set can bear the static tensile force not less than 15N for 15 seconds. (6.3) | Eligible | Eligible | ||||
| Puncture Device Of Bottle Stopper | According to the contract requirements ,Materials for ABS. |
Eligible | Eligible | ||||
| Liquid Medicine Fitter | The infusion set should be equipped with a liquid machine filter | Eligible | Eligible | ||||
| The filtering rate of liquid medicine should be not less than 80%. (6.7) | 98.7% | ||||||
| Flow Speed Of Transfusion (Flow Quantity) | For the infusion set with 20drops/ml, with 1m static press end, the sodium chloride solution flowed should be not less than 1000ml for 10 minutes Unit: ml  (6.10) |
Eligible | Eligible | ||||
| Air Inlet Device (Air Fitter) | The filtering rate against particles 0.5μm in air should be not less than 90%. (6.5.2) | Missing | / | ||||
| Air Inlet Device (Flow Reduction Rate ) | Comparing with vessel with free air input, the flow of effluent should be reduced by 20%. (6.5.5) | Eligible | Eligible | ||||
| Packaging requirements | According to the contract requirements | Eligible | Eligible | ||||
| Flow Adjustor | Materials for ABS. | Eligible | Eligible | ||||
| Soft Pipe (Length) | According to the contract requirements | Eligible | Eligible | ||||
| needle | According to the contract requirements | Eligible | Eligible | ||||
| Testing Results | The seized products meet the requirements of the  contract . |
||||||
INFUSION SET with two plastic inserters two adjustors
Item No: YD180
This is disposable infusion set with two plastic inserters and two adjustors.
Assesoris: Vented spike, dripping champer, roller clamp, solution filter, emulsion tube.
Features:
1) For single use
2) Transparent drip chamber with filter
3) Flexible tubing with standard length of 135cm
4) Thick latex connector
5) Luer lock adapter with hypodermic needle
6) Sterilization: Ethylene-gas-sterilization

| Name of Sample | Disposable Infusion Set with needle | Type | IS-VZ  0.7 | ||||
| LOT NO | 20130618 | Testing Standards | contract requirements | ||||
| Sterilization Lot | 20130618 | Quantity of Tested Batch | 1260000 pcs | ||||
| Quantity Of Sample | 50pcs | Testing Date | SEP 7, 2013 | ||||
| Type | Testing items | Standard Requirement and Standard Term and Conditions | Actual Testing Result | Conclusion | |||
| Biological Properties | Sterility | The infusion set in individual package should be sterile (8.2) | Missing | / | |||
| Pyrogen | Infusion set has no pyrogen (8.3) | Missing | / | ||||
|  Chemical Properties |
Reduction Matter | Difference of volumes of potassium permanganate [ c(kmno4 = 0.002mol/L)] consumed by test liquid and blank liquid should be not more than 2.0 ml (7.1) | 0.5ml | Eligible | |||
| Ph | The PH difference between testing liquid and blank liquid is not more than 1.5 (7.3) | 0.19 | Eligible | ||||
| Ultraviolet Absorbance | The absorbance of testing liquid should be not more than 0.1 (7.5) | 0.046 | Eligible | ||||
| Ethylene Oxide Residuum | The ethylene oxide residuum should be not more than0.5mg; Unit: mg  (7.6) |    0.29 | / | ||||
|  Physical Properties |
Particle Content | For the number of 15-25min particles in 200ml eluent, not more than 1 particle/ml | Eligible | Eligible | |||
| For the number over 25min particles, not more than 0.5 particle/ml (6.1) | Eligible | ||||||
| Sealing Property | No air leakage of infusion set (6.2) | Eligible | Eligible | ||||
| Connection Intensity | Connection of parts for liquid channel of infusion set can bear the static tensile force not less than 15N for 15 seconds. (6.3) | Eligible | Eligible | ||||
| Puncture Device Of Bottle Stopper | According to the contract requirements ,Materials for ABS. |
Eligible | Eligible | ||||
| Liquid Medicine Fitter | The infusion set should be equipped with a liquid machine filter | Eligible | Eligible | ||||
| The filtering rate of liquid medicine should be not less than 80%. (6.7) | 98.7% | ||||||
| Flow Speed Of Transfusion (Flow Quantity) | For the infusion set with 20drops/ml, with 1m static press end, the sodium chloride solution flowed should be not less than 1000ml for 10 minutes Unit: ml  (6.10) |
Eligible | Eligible | ||||
| Air Inlet Device (Air Fitter) | The filtering rate against particles 0.5μm in air should be not less than 90%. (6.5.2) | Missing | / | ||||
| Air Inlet Device (Flow Reduction Rate ) | Comparing with vessel with free air input, the flow of effluent should be reduced by 20%. (6.5.5) | Eligible | Eligible | ||||
| Packaging requirements | According to the contract requirements | Eligible | Eligible | ||||
| Flow Adjustor | Materials for ABS. | Eligible | Eligible | ||||
| Soft Pipe (Length) | According to the contract requirements | Eligible | Eligible | ||||
| needle | According to the contract requirements | Eligible | Eligible | ||||
| Testing Results | The seized products meet the requirements of the  contract . |
||||||
Fresh Ya Pear,Fresh Pear,Ya Pear
Rich Farmer International Trade Co., Ltd. , http://www.jnfreshfruit.com