In short: the lower the sterilization temperature, the longer the sterilization time required; the higher the sterilization temperature, the shorter the sterilization time required
The time of pressure steam sterilization should be counted from the time when the sterilization chamber reaches the sterilization required temperature until the sterilization is completed.
Total time includes:
1, thermal penetration time
That is, the time from the sterilization cabinet to the sterilization temperature to the center of the disinfection article also reaches the sterilization temperature. The length of time depends on the nature of the sterilized item, the size of the package, the placement and the degree of air venting in the autoclave and the type of sterilizer.
2, disinfection maintenance time
That is, the time required to kill the microorganisms is generally expressed by the time required to kill the cells of the Thermomyces faecalis. It takes 30 minutes at 115 ° C, 12 minutes at 121 ° C, and 2 minutes at 132 ° C.
3. Safe time
Generally, it is half of the maintenance time, and its length depends on the disinfecting items. Sterilization of easily conductive metal equipment does not require safe time.
The data provided in the table below is available for reference in actual work.
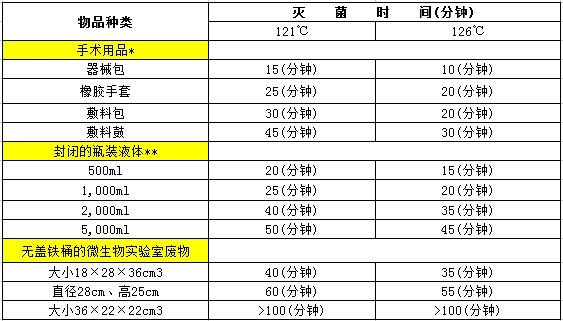
Note:
*Packing volume < 30×30×30cm3
** For viscous liquids, such as agar medium, extend the time for 5 to 10 minutes.
1) Generally, the time required for the vented autoclave is: 30 minutes for 115°C, 15 minutes for 121°C, and 10 minutes for 126°C.
2)     115 ° C is commonly used for sterilization in the pharmaceutical industry. In the microbiology laboratory, some sugar-containing media are also sterilized at 115 ° C for 30 minutes because the sugar will decompose at higher temperatures.
3)     121 ° C ~ 126 ° C is often used for sterilization in medical and epidemic prevention work.
The approximate number of knowledge sterilization time described above, and how much time is used in the single sterilization, needs to be determined according to the type of the article, the size of the package, the placement, and the performance of the sterilizer.
We, Jiangsu YanFang Medical Technology Co., Ltd, commenced our medical gloves manufacturing in 2020. Currently, we possess a total of 12 high-capacity NBR Glove Dipping Production Lines.
Likewise, we are not only certified with ISO9001, ISO13485 but also fully complied with the essential USFDA, CE Compliances, as well as obtaining relevant accreditation of FDA510K, EN455, and EN374.
Nonetheless, our NBR Examination, Chemotherapy, and Food Grade products are being well established in both US and Europe markets.
We look forward to cooperating and working closely with our valuable customers and stakeholders, who are seeking long-term business relationships in high-quality NBR glove supplies.
Disposable Nitrile Gloves,Household Nitrile Gloves,Safety Nitrile Gloves,Powder Free Nitrile Gloves
Jiangsu Yanfang Medical Technology Co.,Ltd. , https://www.yanfangchina.com