The most widely recognized transfection technology in the world, targeting almost all difficult-to-transfect cell lines or primary cells such as immune cells, nerve cells, and stem cells, as well as suspension cells. ——Amaxa brand Nucleofector nuclear transfection technology
Amaxa® Nucleofector® technology is a patented innovation of Lonza (formerly Amaxa), which combines traditional electroporation technology with cell-specific nuclear transfection fluids by adjusting the optimized electrical parameters (memory transfection procedures). The source gene is introduced into the cytoplasm and nucleus of primary cells and cell lines.
Founded in 1998, Amaxa is a fast-growing biotechnology team in Germany dedicated to the development of a highly efficient, non-viral gene transfection technology, Nucleofector. Based on Nucleofector technology and successful financing, Amaxa has grown rapidly from the first two to more than 80 technicians and quickly became one of the leading companies in the field of non-viral gene transfection technology.
LONZA (formerly Amaxa) Nucleofector Transfection Reagent and Nucleic Acid Transfection Apparatus China Agent: Murray (Shanghai) Biotechnology Co., Ltd.
Working principle
Amaxa® Nucleofector® technology is a patented innovation of Lonza (formerly Amaxa), which combines traditional electroporation technology with cell-specific nuclear transfection fluids by adjusting the optimized electrical parameters (memory transfection procedures). The source gene is introduced into the cytoplasm and nucleus of primary cells and cell lines. It differs from traditional electroporators in that it allows direct introduction of foreign genes into the nucleus and that cell survival rates are much higher than electroporation.
Nucleofector technology is unique in that it directly transfects DNA into the nucleus, thus enabling efficient transfection of primary cells that have been plagued by cell division limitations. With the help of the Nucleofector nucleic acid transfection device, you can easily achieve high-efficiency gene transfection rates of more than 90% in the fields of cancer research, immunology, tissue engineering and cardiovascular disease research. Nucleofector nucleic acid transfection technology is particularly suitable for transfection of primary cells and cell lines that are difficult to transfect, such as T cells and PC-12 cell lines.
The Nucleofector kit contains two solutions: Nucleofector solution and additives. Both are specifically developed for each cell type. They provide a cell-friendly environment during nuclear transfection, supporting DNA directly to the nucleus. The Nucleofector solution works only when used in conjunction with the Nucleofector unit.
Product advantages
1. Cell-free mitosis, suitable for difficult to transfect cells such as suspension cells and primary cells .
2. High transfection efficiency, direct transfer of foreign genes into the nucleus.
In some cells, Nucleofector can achieve a transfection efficiency of 90%, which is as effective as viral infection. Nucleofector transports DNA directly into the nucleus, so gene expression can begin immediately, and the entire process of transfection and analysis can be completed in a matter of hours. For cell lines, it can be analyzed in 2-6 hours, for primary cells. It takes 4-16 hours.
3. Can be transfected with DNA, RNA or siRNA.
4. The operation is simple and convenient , Nucleofector transfection technology can be completed in less than one hour.
5. The most widely used cell types, the globally shared cell transfection database is expanding. At present, more than 1,200 cell lines have been efficiently transfected , and more than 130 primary cells have been successfully transfected .
6. Maintain cell physiological function .
Nucleofector cell nuclear transfection technology steps
The entire operation takes only four steps and is very simple and efficient.
Step 1: Treat cells, count accurately
Step 2: Mix the DNA, transfection solution, resuspend the cells, and add the transfection cup
Step 3: Put the transfection cup into the transfection apparatus, press the button to select the corresponding program, press the "start" button to start transfection, about 10 seconds
Step 4: Remove the cells and continue to culture
The entire operation takes only four steps and is very simple and efficient.
Step 1: Treat cells, count accurately
Step 2: Mix the DNA, transfection solution, resuspend the cells, and add the transfection cup
Step 3: Put the transfection cup into the transfection apparatus, press the button to select the corresponding program, press the "start" button to start transfection, about 10 seconds
Step 4: Remove the cells and continue to culture
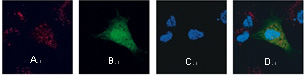
A foreign gene is directly turned into the nucleus. Normal human dermal fibroblasts –neonatal were nucleofected with 2.5 μg TMR-labeled plasmid DNA encoding eGFP. Fixation of cells was performed after 2 hours with 3.5 % PFA. TMR label is shown in (A), GFP fluorescence in (B), DAPI nuclear Staining in (C) and a merge of all three fluorescent labels in (D).
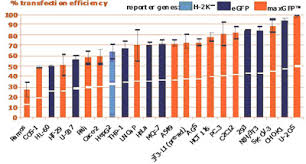
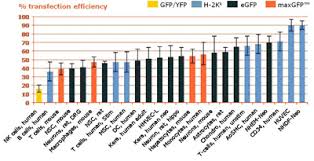
Efficient transfection of the widest range of cell types
Extended reading
(a) The application of Lonza Nucleofector® Technology in neurobiology
—— Nucleofector® technology enables you to transfect nerve cells of different species and origins stably and efficiently
Traditional lipofection methods and electroporation transfection techniques are not effective in transfecting neuronal cells. Because Lonza's Amaxa Nucleofector® electroscopy instrument uses the patented Nucleofector® technology to introduce foreign genes directly into the nucleus, overcoming liposome transfection and ordinary electroporation must rely on cell division to make it possible to exogenous Defects in gene expression into the nucleus. If the Nucleofector® 4D Y Unit is used to rotate the cells in situ, even the primary neurons that are transfected with cryopreserved can achieve the same effect as freshly extracted neurons.
(B) the application of Lonza NucleofectorTM technology in diabetes research
At this stage, the research model of diabetes mainly includes living model and cell model of diabetic rats. The cell model mainly studies the formation, pathogenesis and genetic mechanism of diabetes from the molecular level, and strives to treat diabetes by molecular means, especially for the treatment of family-aged diabetes. In modern medical and biological research, cells commonly used in diabetes research include THP-1, murine primary CD4+ T cells, primary aortic endothelial cells, mouse islet cell line (MIN6), Hela cells, and coronary arteries. Smooth muscle cells (CASMCs), HL60, human retinal pigment epithelial cell line (ARPE-19), human PBMCs cells, RBL-2H3 and other cells. In cell-based studies, researchers need to efficiently transfer foreign substances of interest (plasmid DNA, siRNA, etc.) into cells, which have reached the goal of interfering with the target gene; Cells are very difficult to transfect cells. It is difficult to transfer foreign genes into cells efficiently by conventional transfection methods. Therefore, it is very important to choose a suitable cell transfection method for the smooth development of the experiment. In the previous diabetes research, in order to be able to efficiently transfect cells, many scientists have chosen Lonza nuclear transfection technology as a method and means of cell transfection, achieving efficient transfection of cells and achieving satisfactory results. Experimental results.
references
1. Marina Cardellini et al. used coronary artery smooth muscle cells (CASMCs) as a model to study the regulation of TIMP3 expression in atherosclerotic plaques of type II diabetes patients by Lonza nuclear transfection. The results showed that inhibition of SirT1 activity in CASMCs down-regulated the expression of TIMP3, while overexpression of SirT1 up-regulated the expression of TIMP3.
2. Serve Olieslagers uses human siRNA knockout technology (Lonza nuclear transfection system) to knock out SMAD2 and SMAD3. The cell survival rate after electroporation is >90%. The results of the experiment showed that SMAD2 and SMAD3 did not affect TGF-β1-mediated monocyte migration ability.
3. Suseela Srinivasan et al. used mouse primary CD4+ T cells as a model to study whether S1P can reduce the expression of HIF1α I.1 and CD69 by reducing the expression of HIF1α I.1 and CD69 by using Lonza nuclear transfection (siRNA). The activity of T cells, in turn, explores the therapeutic potential of S1P to reduce inflammation in people with I diabetes. The results showed that S1P can significantly up-regulate HIF1αI.1, thereby down-regulating the expression of IFN- and CD69, and reducing the activity of human CD4+ T cells in type I diabetes, with certain potential therapeutic ability.
4.Tian et al. used mouse primary aortic endothelial cells as a model to study the interaction between Akt signaling pathway and GW1516 by Lonza nuclear transfection (transfection efficiency 70%). The experimental results show that inhibition of Akt activity reduces the activation of GW1516 in mouse primary aortic endothelial cells.
Intza Garin et al. used Hela cells as a model and used Lonza nuclear transfection technology to transfer two important INF recessive mutation genes into Hela cells to study whether the mutant gene has an effect on insulin synthesis. The results of the experiment showed that the insulin content in the cells transferred to the recessive mutant gene was reduced by 86% and 79%, respectively, compared with the cells transfected with the normal INF gene. Therefore, Intza Garin et al. believe that individuals with recessively mutated INF genes will reduce insulin synthesis and induce neonatal transient diabetes.
6. Niels Engberding [6] used Sprague-Duller rat primary vascular smooth muscle cells (VSMCs) as a model, and overexpressed and down-regulated IGF1Rs by adenovirus infection and Lonza nuclear transfection (RNAi), respectively. To study the effect of changes in the expression of IGF1Rs on the insulin sensitivity of VSMCs. The results show that overexpression of IGF1Rs reduces insulin-mediated Akt phosphorylation; downregulation of IGF1Rs enhances insulin-mediated phosphorylation of IRβ and enhances glucose uptake by cells.
7. Jamie Cantrell Stanford et al. used the siRNA technology (Lonza nuclear transfection system) to knock out sphingosine kinase 2 (SphK2) and Sgpp1 (S1P phosphatase), respectively, using the MIN6 cell line as a model. The regulation of aminoethanol kinase (S1P) on glucose-stimulated insulin secretion. The experimental results show that S1P plays a key role in the regulation of glucose-stimulated insulin secretion.
Murray Bio is an authorized agent of Lonza Cell Transfection Reagents. You are welcome to consult and order.

support hotline
Pre-sales advice:
ordering product:
after sales support:
Murray's official website:
Vegetarians Size 00 Empty Capsule
Vegetarians Size 00 Empty Capsule,Gelatin Empty Pills Capsules Vegan,Colorful Vegan Empty Pills Capsules,Size 00 Empty Pills Capsules Vegan
Ningbo Jiangnan Capsule Co., Ltd. , https://www.ningbocapsule.com