On November 28th, the National Health and Family Planning Commission and the State Food and Drug Administration jointly issued the second batch of stem cell clinical research filing agencies. A total of 72 medical institutions were selected. Previously, on October 30, 2016, the National Health and Family Planning Commission and the State Food and Drug Administration announced the first batch of 30 clinical research institutions for stem cells, which means that the above 102 institutions will be able to carry out clinical research related to stem cell therapy.
According to the previously published "Methods for the Management of Stem Cell Clinical Research (Trial)", institutions conducting stem cell clinical research must have the following seven conditions:
Stem cell clinical research institutions should have the following conditions:
(1) Class III A hospitals have medical and therapeutic subjects corresponding to the clinical research of stem cells.
(2) Obtaining the qualifications of the drug clinical trial institutions of relevant professions according to law.
(3) It has strong comprehensive ability in medical treatment, teaching and scientific research, and undertakes major research projects in the field of stem cell research, and has legal support, relatively stable and sufficient project research funding support.
(4) It has complete stem cell quality control conditions, comprehensive stem cell clinical research quality management system and independent stem cell clinical research quality assurance department; establishes stem cell preparation quality attorney system; has complete stem cell preparation preparation and clinical research process quality management And risk control procedures and related documents (including quality management manual, clinical research work procedures, standard operating procedures and test records, etc.); with a stem cell clinical research audit system, including qualified internal auditors and internal audit, external audit system.
(5) The person in charge of the stem cell clinical research project and the recipient of the quality of the preparation shall be formally authorized by the main person in charge of the organization, have a senior professional technical title, and have a good scientific research reputation. The main researchers were trained in the Quality Control Practice for Drug Clinical Trials (GCP) and obtained the corresponding qualifications. The institution shall allocate sufficient qualified human resources to carry out corresponding stem cell clinical research, formulate and implement a training program for stem cell clinical researchers, and monitor the training effect.
(6) Academic committees and ethics committees composed of high-level experts who are compatible with the clinical research of stem cells.
(7) Management mechanisms that prevent the risk of clinical research on stem cells and measures to deal with adverse reactions and adverse events.
At the same time, the "Measures" clarify that medical institutions that carry out clinical research on stem cells (hereinafter referred to as institutions) are responsible for the quality management of stem cell preparations and clinical research. The institution shall conduct a project review, registration and process supervision of the stem cell clinical research project, and conduct quality management and risk management and control over the whole process of stem cell preparation and clinical research.
In addition, the "Measures" also clarify that medical institutions may not charge the patients for stem cell clinical research related expenses, and may not publish or disguise the publication of stem cell clinical research advertisements. Conducting stem cell clinical research must follow the principles of science, regulation, and openness, and must follow the principles of ethics and adequate protection of the rights of subjects.
Attached to the second batch of stem cell clinical research filing agencies
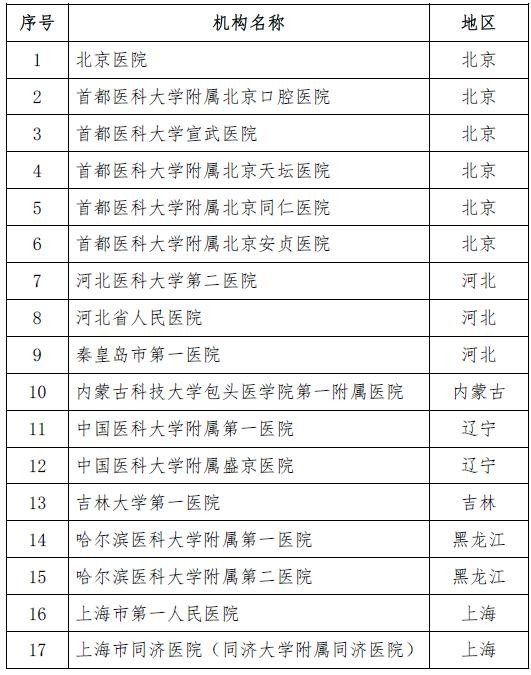
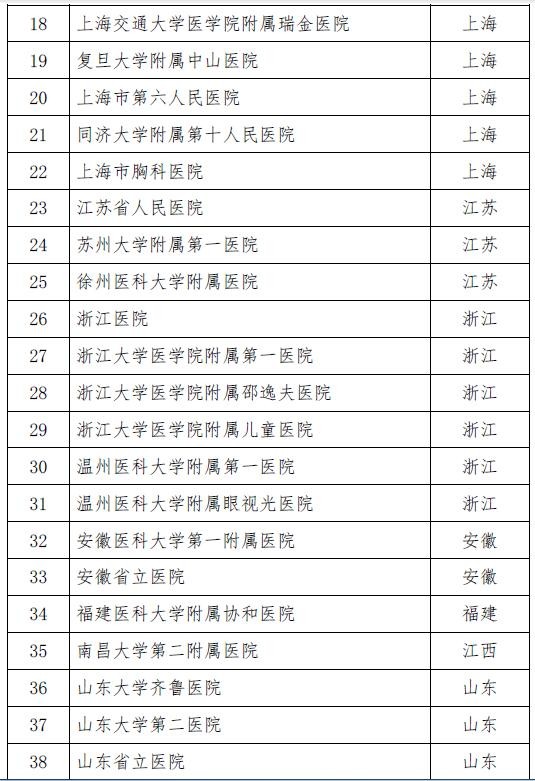
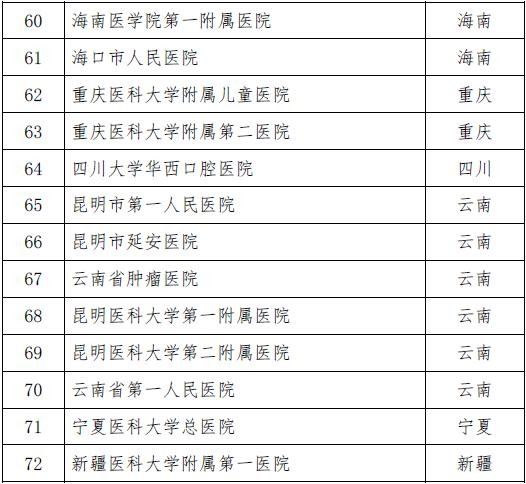
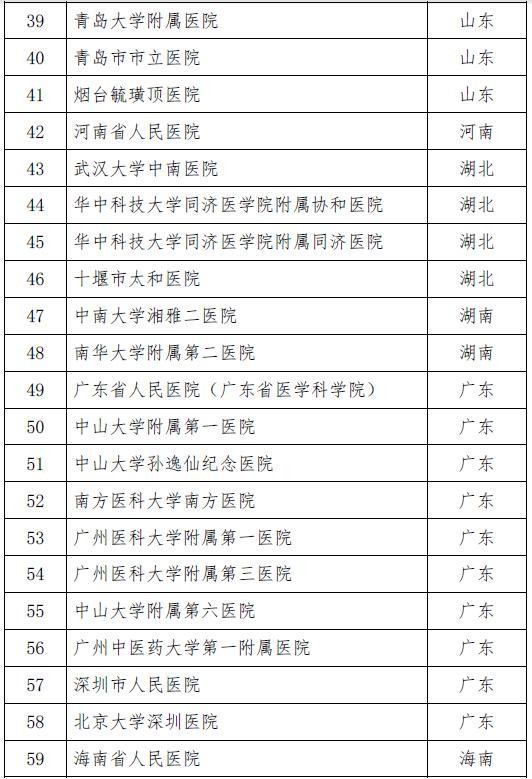
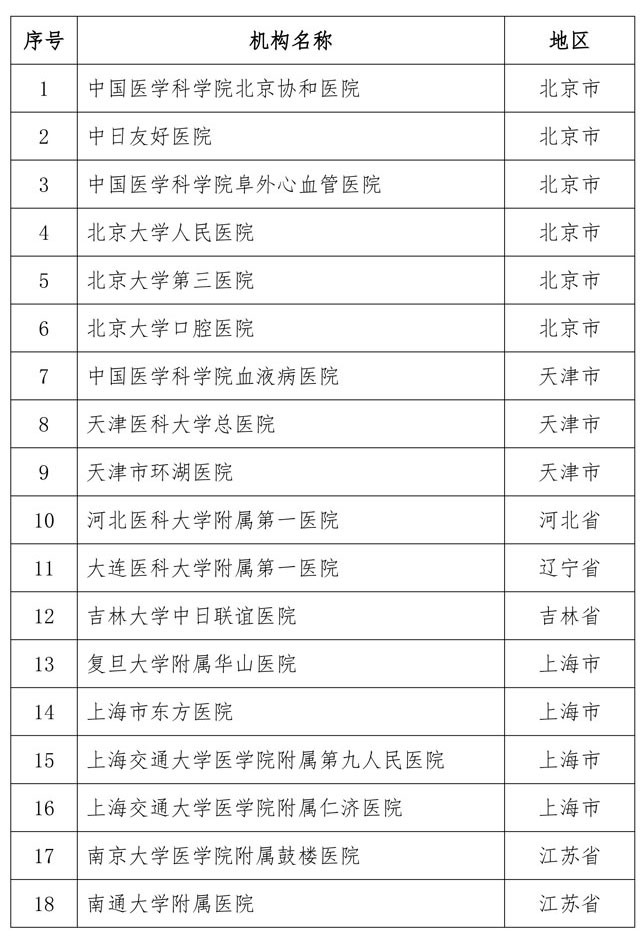
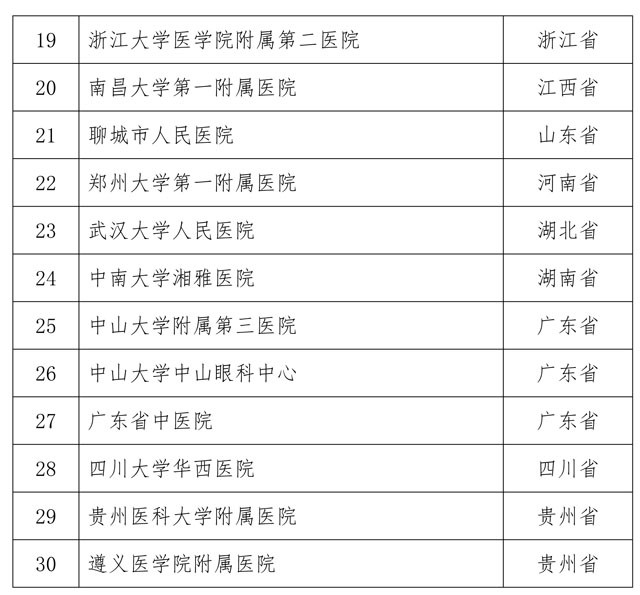
Attached to the first batch of stem cell clinical research filing agencies
Shanghai Enjosim Medical Technology Co., Ltd, Jiangsu Enjosim Medical Technology Co., Ltd , https://www.enjosimmedical.com